Molecular control of neural crest cell patterning
Neural crest cells (NCCs) arise at the junction between the neural and non-neural ectoderm before moving ventrally around the embryo along the entire rostro-caudal axis. Cranial NCCs form most of the bone and cartilage in the face, explaining why defects in early NCC patterning are so devastating to human facial development. Our lab uses a number of cutting edge molecular and cellular techniques and approaches in both mouse and zebrafish models to dissect signaling networks that decide the fate of NCCs. We then use this information to better understand the basis for human birth defect syndromes affecting the face for which a genetic basis has not been established.
Regulation of neural crest cell identity
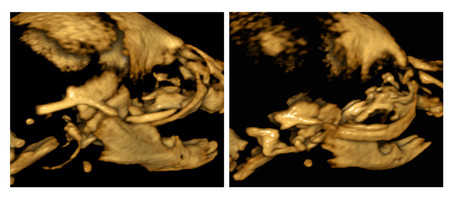
The fate of cranial NCCs in the jaw region is decided by the 21 amino acid molecule, Endothelin1 (EDN1). Restricted to the lower jaw region, EDN1 drives signaling in NCCs that establish lower jaw identity and ensures formation of lower jaw and middle ear structures. Our lab studies how disruption of Edn1 signaling leads to changes in jaw structures and how EDN1 and JAGGED/NOTCH signaling antagonize each other to form a functional jaw joint. We are particularly interested in specific molecular events downstream of EDN1 and JAGGED/NOTCH signaling that appear to be regulated by both microRNAs and long non-coding RNAs. Tissue-specific inactivation of these genes in mice will provide new clues into how facial morphogenesis is regulated.
The role of bHLH proteins in facial morphogenesis
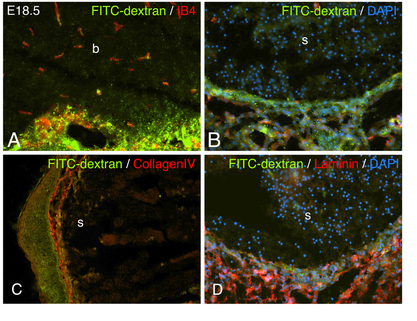
Basic helix-loop-helix proteins play important roles in the development of many tissues. In the face, the TWIST family members HAND2 and TWIST1 are both involved in lower jaw development. Deletion of either gene in the early first arch leads to mandibular defects. However, each is expressed in different domains of the mandibular pharyngeal arch and only overlap within a small domain. This domain appears to regulate the interaction of NCCs and the surrounding vasculature, an aspect of facial development that is poorly understood. With conditional alleles for both genes, we are poised to decipher critical events required for facial development and to understand how these interactions go awry in human development.
Nkx proteins and the patterning zebrafish NCCs
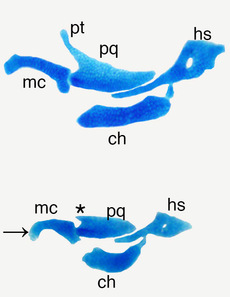
Nkx2.5 is required to define ventricular cells during early zebrafish heart development. Loss of Nkx2.5 and Nkx2.7 leads to a transformation of ventricular cells into atrial cells. We are interested to learn if these Nkx proteins also provide patterning or survival cues for cranial NCCs. Preliminary evidence suggests that these molecules work upstream of Edn1, but how such a function would occur is unknown. We currently have zebrafish mutants for several different nkx genes in addition to reporter and rescue alleles and are using these to tease apart the functions of these genes in facial development.
